Spravato Program
The Rochester Center for Behavioral Medicine is pleased to offer SPRAVATO® (esketamine), manufactured by Janssen Pharmaceuticals. This medication is used adjunctively with antidepressant medications for treatment-resistant depression. Our medical director, Dr. Joel Young, was a primary investigator during the SPRAVATO® clinical trial.
This page presents extensive information regarding SPRAVATO® and our state-of-the-art SPRAVATO® Treatment Program at RCBM. In our frequently asked questions (FAQs) below, you will learn about SPRAVATO®, who can and cannot use SPRAVATO®, what to expect throughout the treatment process, and insurance coverage, along with the effectiveness of SPRAVATO®.
Referral forms are located below the FAQs.
Doctors answer patients’ top 9 questions about SPRAVATO®.
Watch this video in order to learn more about one individual's personal experience with SPRAVATO®
FAQs
About SPRAVATO®
Major Depressive Disorder (MDD) is one of the most common mental health disorders in the United States. Treatments such as psychotherapy, medication management, and lifestyle changes may help relieve symptoms. However, other individuals may have Treatment Depression (TRD) and continue to experience symptoms such as persistent feelings of sadness, sleep disturbances, low energy, and thoughts of death or suicide despite trying multiple treatments.
Taken along with an oral antidepressant, SPRAVATO® ® is the first nasal spray medication specifically for adults with treatment-resistant depression.
Taken along with an oral antidepressant, SPRAVATO® ® is the first nasal spray medication that treats depressive symptoms in adults with major depressive disorder with suicidal thoughts or actions.
SPRAVATO® ® was approved by the U.S. Food & Drug Administration on March 5, 2019, and is only available to patients at certified treatment centers in the United States, such as Rochester Center for Behavioral Medicine (RCBM).
SPRAVATO® ® is a prescription medication, used along with an antidepressant taken by mouth, for TRD in adults.
This treatment is approved for adults ages 18 and older.
Distribution and administration of SPRAVATO®
SPRAVATO® ® is available only through a restricted distribution program called the SPRAVATO® ® REMS (Risk Evaluation and Mitigation Strategy). A REMS program is in place to ensure the safety of all patients who are treated with SPRAVATO® ®. The Rochester Center for Behavioral Medicine’s SPRAVATO® Program operates underneath the REMS program for service implementation at our office.
The goals of the REMS are to mitigate the risks of serious adverse outcomes resulting from sedation and dissociation caused by SPRAVATO® ® administration and abuse and misuse of SPRAVATO® ® by ensuring SPRAVATO® ® is only dispensed and administered to patients in medically supervised healthcare settings that monitor these patients, ensuring pharmacies and healthcare settings that dispense SPRAVATO® ® are REMS certified, ensuring patients are informed about serious adverse outcomes from dissociation and sedation and the need for monitoring, and enrolling all patients who receive treatment in an outpatient healthcare setting in a REMS registry to characterize the risks further and support safe use.
What is the difference between ketamine and esketamine (SPRAVATO®)?
Ketamine is a generic drug used off-label for depression, while SPRAVATO® is a brand-name nasal spray derived from ketamine and FDA-approved for treatment-resistant depression.While SPRAVATO® and Ketamine are chemically related, SPRAVATO® is not the same as IV Ketamine. Only SPRAVATO® has undergone extensive controlled clinical trials that informed the FDA approval of the medicine for use in adults with treatment-resistant depression and to treat depressive symptoms in adults with major depressive disorder with acute suicidal ideation or behavior.
Esketamine is made from a drug called ketamine, an anesthetic that has also been used for many years to treat depression. Because Esketamine is more potent, it can be used at a lower dose and has fewer side effects. Esketamine has earned FDA approval specifically for use as a nasal spray for those with treatment-resistant depression. Because the FDA approves it, it’s more likely that insurance companies will cover the treatment.
For more information on Ketamine, please review:
Ketamine for Treatment-Resistant Depression and Related Conditions: A Review of a Novel and Needed Treatment Option by Benjamin Young, MD
Who cannot use SPRAVATO®?
You cannot use SPRAVATO® if you…
-Have a Blood Vessel Disease
-Have an abnormal connection between your blood vessels (arteriovenous malformation)
-Have a history of bleeding in the brain (intracerebral hemorrhage)
-Are allergic to Esketamine, Ketamine
-Are Lactating, Pregnant, or planning to become pregnant
-Active substance use disorder
-HIV
Precautions: If you are interested in SPRAVATO®, talk to your healthcare provider if you have the following medical conditions…
- Hypertension (High blood pressure)
- History of Stroke or Heart Attack
- Heart Valve Disease
- History of Brain Injury
- Liver Disease
- History of Psychosis
- History of Seizures
- History of Substance Abuse
- On MAO inhibitors
Does my insurance cover SPRAVATO®?
- Insurance coverage varies based on the individual health plan. We encourage you to check your insurance benefits for SPRAVATO® coverage, including co-pays and deductibles, along with whether SPRAVATO® falls under your medical or pharmacy benefits. The RCBM SPRAVATO® Program does not participate with TriCare, UMR, Blue Care Network, Humana, United Healthcare, or Medicaid. If you have one of these health plans, please visit SPRAVATO®.com to find a SPRAVATO® center that does take your insurance.
- All insurances require pre-authorization and continued authorization for SPRAVATO® treatment, while each insurance company has different criteria for authorization of SPRAVATO®. This includes a history of inadequate response to several different classes of anti-depressant medications with a trial of a minimum of 8 - 12 weeks each, along with augmentation strategies.
- Your healthcare provider must refer you for SPRAVATO® treatment and document the medical necessity of SPRAVATO® based on the required criteria.
- If you have a commercial health plan, SPRAVATO with me is available to help you navigate access and affordability.
SPRAVATO® referral timeline
The following is a timeline of what you should expect from referral through the course of treatment:
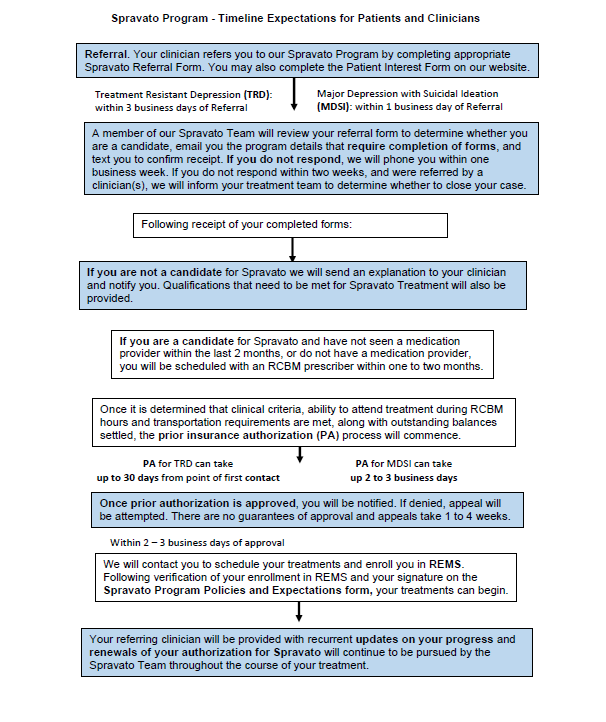

How effective is SPRAVATO® treatment?
In clinical studies, patients who achieved stable remission with SPRAVATO®, augmented with an antidepressant, showed a 51% decrease in risk of relapse. In addition, there was a 70% risk reduction for depressive symptoms among those who achieved a stable response compared to an antidepressant alone.
For individuals with TRD
In clinical studies, improvement of depressive symptoms was demonstrated at four weeks by SPRAVATO® ® plus an oral antidepressant, compared with placebo nasal spray plus an oral antidepressant. Not all patients will respond to SPRAVATO® ®.
For individuals with MDSI
In clinical studies, those who took SPRAVATO® ® and an oral antidepressant experienced a greater reduction of depressive symptoms at 24 hours compared to those who took a placebo plus an oral antidepressant. Further reductions were seen consistently through four weeks (25 days) of treatment, so it is important to follow the treatment plan that you and your healthcare provider have made. Not all patients will respond to SPRAVATO®
Please read our Full Information SPRAVATO® Pamphlet before submitting a patient interest or Healthcare provider referral form.
Patients must have an active diagnosis of either Treatment Resistant Depression (TRD) or Major Depression with Acute Suicidal Ideation (MDSI) in order to be eligible for treatment with SPRAVATO®.
For more information on SPRAVATO®
In addition, watch this video that highlights additional common questions for those interested in Spravato treatment.
Please note important requirements for treatment in our program prior to completing the interest form or referral forms:
- Patients must have an active diagnosis of either Treatment Resistant Depression (TRD) or Major Depression with Acute Suicidal Ideation (MDSI) in order to be eligible for treatment with Spravato.
- Business hours are between 9:00 am – 5:00 pm, with last treatment appointment scheduled at 3:00 pm.
- Appointments are at least two hours in length and require a twice per week regimen for the first four weeks, followed by once per week for one month. Continued dosing based on response to treatment.
- Patients must have a driver to and from each appointment and are not allowed to resume driving until the next morning.
For patients who have been seen by an RCBM provider within the past 6 months, please DO NOT complete the forms below. Please discuss your interest with your RCBM provider at your next appointment. Thank you!
Patient Interest Form for Spravato Treatment:
Open FormHealthcare Professional Referral Form:
Open FormPlease make sure to read the above information. If you have additional questions or concerns, prior to completing the respective interest form, please feel free to email esketamine@rcbm.net.












